lab work on a research vessel

Live counting of protists - an interview with Manon Hohlfeld
Manon Hohlfeld is a PhD student at the University of Cologne. As part of the MGF Baltic Sea project, she studies colorless protists - small microorganisms that consist of only a single cell and have a nucleus. These include, for example, flagellates, amoebae, ciliates (ciliates) or foraminifera (chamberlings). During the expedition with the research vessel Elisabeth Mann Borgese (EMB238), Manon counted protists alive. How this works on board and whether the swaying of the ship interferes with counting, she tells here.
Interviewer: Manon, in order for you to be able to count on the ship at all, a lot of materials and equipment had to be taken on board. Why is it better to count protists on board rather than in the laboratory at the University of Cologne?
Manon: Yes, we need a lot of things for the live counts. The most important things are a light microscope and microscopy accessories such as slides and pipettes to make the small organisms visible to the human eye. In addition, so-called autoclaved i.e. sterilized seawater is needed, but alternatively filtered seawater. Since we also do the autoclaving on board, we of course also needed the necessary material for this, such as a pressure cooker. For filtering we use a filter with a pore size of 0.2 µm - that is one fifth of a thousandth of a millimeter!
Nevertheless, the live counts can actually only be done on board, because the time until we are back in Cologne is simply too long - by then the live samples have changed a lot in terms of their species composition. After sampling, the protists from the samples are cultured, i.e. conditions are artificially created to ensure growth of the organisms. In culture, a few species then prevail and dominate, while other species disappear completely because they do not cope well with competition or with the conditions in culture. However, there is also the possibility of fixing and staining the samples with chemicals, in which case they can be counted later, but then it is not possible to determine which species they are. Fixation causes protists to change shape and often lose flagella - thread-like appendages on the surface of individual cells used for locomotion. The problem with this is that a large proportion of protists can only be determined by their movements. So we need the live counts to be able to assign species. Therefore, we always use both methods, live counts and fixed counts, because both methods have the advantages and disadvantages described before.

Interviewer: How does a sampling procedure work to get protists?
Manon: We have taken samples with the multicorer, a gear that retrieves sediment cores and bottom water from the seafloor, and we have cut the obtained cores into different layers. A defined volume (a small spoonful of sediment) is then taken from the sediment samples and diluted with filtered or autoclaved seawater. Once the samples have been prepared in this way, I look at them under a light microscope (several drops between 1 and 5 µl) and count the protists present. These are mainly flagellates, amoebae and ciliates. However, the procedure is not for someone who gets seasick quickly. The sample under the microscope moves with every wave. But on the EMB238 cruise we were really lucky with the weather and one hardly noticed that one was on a ship at all.
Interviewer: Are there any initial (surprising) findings that you have already made on board?
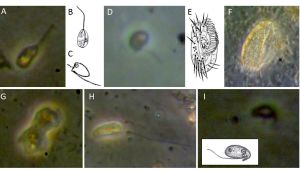
Manon: The live counts on the EMB238 cruise were already a bit surprising for me, because until this cruise I had mainly studied samples from the deep sea, mainly from the Atlantic. The Baltic Sea, with only 2-20‰ salinity, has much lower salinity than the Atlantic, for example, which has 35‰, so one of the species we found on the EMB238 cruise were those that are normally found mainly in freshwater. However, I also found typical marine benthic protists, especially amoebae (see image G), ancyromonads (image C and D), euglenids (image A, B) and bodonids (image H and I), as well as ciliates (image E and F), which are extremely rarely found in the deep sea, for example.
Interviewer: What are the next steps in the evaluation?
Manon: On board I have already completed the live counts and fixed and stained samples. For staining, we use DAPI - which is a fluorescent dye used in fluorescence microscopy to label DNA. I also have frozen samples for molecular genetic studies and set up live cultures. Back in Cologne, we were mainly focused on further cultivation, as this had to happen quickly. In the meantime, we are isolating species so that they can survive in cultures in the long term and we can characterize and determine them morphologically and phylogenetically. Next, we will then conduct metabarcoding sequencing on the frozen samples. That means we sequence a certain so-called marker region on a certain gene region for all organism in the sample to get an overview of the whole protist community. We will then compare the community in the marine protected area Fehmarnbelt with the community in the control region where a lot of mobile bottom-contact fishing takes place.
Interviewer: Thank you very much for the informative interview!

