Ocean interfaces under global changes –
Exploring the fate of the Amazon River plume
08.04.2021 – On the importance of studying major freshwater loads in the Ocean
This is day 8 out of 10 days of quarantine we have to go through before the cruise. While waiting in our hotel rooms for the journey to start, we are getting more and more excited to finally set foot on the gangway of R/V Meteor. In this entry, we would like to introduce our study area a bit more to you - read along why the Amazon is so fascinating to us.
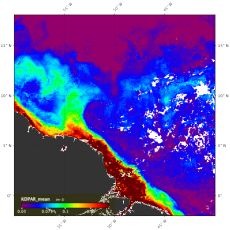
We often refer to the Amazon Rainforest as the “lungs of the Earth” because of its key role in the global carbon cycle, contributing to about 15% of the global photosynthesis1: by “breathing”, trees absorb CO2 and convert it into organic material such as leaves, humus and wood2. The Amazon River basin receives a tremendous amount of precipitation of ~2000 mm of rainfall per year3. Combined with the high ambient humidity of the region, it feeds the Amazon River4,5, which contributes to almost 20% of global river freshwater input to the ocean5. The colossal load of water transported by the Amazon River drags large portions of the organic material and nutrients produced by the forest, enriching the Western Tropical North Atlantic (WTNA)6,7.
As phytoplankton growth is limited by nutrients, the river water enhances primary production in the WTNA, hence increases the amount of CO2 that is taken up during photosynthesis. When the plume mixes with the WTNA (mesohaline region of the plume) the nutrient content is dominated by silicate and phosphate and depleted in nitrates8. It is therefore hard for common phytoplankton to grow. But some phytoplankton species (diazotrophs) are able to find their nitrogen in another pool: dissolved di-nitrogen (N2). This form of N is not accessible to other phytoplankton. Therefore, nitrogen fixers play a key role in primary production in the WTNA, as they profit from silicate and phosphate delivered by the Amazon River, but do not depend on nitrogen in surrounding waters. Alone, nitrogen fixers (diazotrophs) are not responsible for huge CO2 storage. However, they fertilize the rest of the ARP, as they release nitrogen species available to other phytoplankton communities throughout their life or after their decomposition.
These specific conditions lead to an interesting symbiosis between a microalgae, or phytoplankton (diatom) and a bacteria capable of fixing atmospheric nitrogen (diazotroph)9. The diatom feeds on the plume’s silicates and phosphates while getting its nitrogen from the diazotrophs it is associated with. Frequent Diatom Diazotroph Associations (DDAs) blooms may foster massive CO2 drawdown directly or by fertilizing other phytoplankton with biologically available nitrogen9–12. The Amazon River’s physical and biogeochemical properties shape the phytoplankton community structure in the WTNA6,8,13,14, intensifying or weakening the CO2 sink created by the mixing between the Amazon River Plume and the ocean12,15,16.
Human activities are changing the face of Amazonia, impacting its global carbon balance17, and hydrological cycle18. Deforestation releases the carbon stored within the trees back into the atmosphere, dominating biotic atmospheric CO219, decreasing the forest’s CO2 storage capacity by 40 % (Carbon biological pump)20, triggering warming21. Deforestation has also affected Amazonia hydroclimate18,22,23. Several climate scenarios predicted a disturbance of the natural El Niño Southern Oscillation (ENSO), a climatic phenomenon, leading to more frequent and severe occurrence of ENSO-related droughts24 and floods14,18,25. Prolonged drought could lead to a massive reduction of the river runoff26. Changes in the Amazon could trigger really broad consequences, with cooler and drier winters in northern Europe and eastern Canada and warmer and wetter ones in southern Europe and the eastern United States. The induced warming over the Arctic would lead to a reduction of local sea-ice extent and enhanced high latitude river runoff27. Yet, changes in the physical and biogeochemical proprieties of the plume13,14 following these changes are still poorly constrained.
Understanding the Amazon River Plume’s carbon biological pump is therefore of major concern. As the most important sinks of carbon have been found in the nitrogen-limited zone, having a better constrain of the use and transformation of nitrogen in this complex system is relevant for our understanding of the future of planet Earth. For this purpose, the German Research Foundation (DFG) funded MeNARP Project (Metabolism of Nitrogen in the ARP and Tropical North Atlantic) studies the impact of riverine N on cycling and turnover of N and carbon and functional biodiversity of higher trophic levels.
In the next entry, we will talk a bit about our quarantine experiences and our “journey before the journey”.
Text: Choisnard N., Umbricht J., Voss M. (all IOW)
| Expedition: | M174 |
| Mission: | MeNARP |
| Start: | 12.04.2021 - Las Palmas |
| Destination: | 31.05.2021 - Emden |
References:
- Field, C. B., Behrenfeld, M. J., Randerson, J. T. & Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 281, 237–240 (1998).
- Raich, J. W. & Potter, C. S. Global patterns of carbon dioxide emissions from soils. Global Biogeochemical Cycles 9, 23–36 (1995).
- Henderson, A. The palms of the Amazon. The palms of the Amazon. (1995).
- Recycling of water in the Amazon Basin: An isotopic study - Salati - 1979 - Water Resources Research - Wiley Online Library. https://agupubs.onlinelibrary.wiley.com/doi/epdf/10.1029/WR015i005p01250.
- Dai, A. & Trenberth, K. E. Estimates of Freshwater Discharge from Continents: Latitudinal and Seasonal Variations. JOURNAL OF HYDROMETEOROLOGY 3, 28 (2002).
- Smith, W. O. & Demaster, D. J. Phytoplankton biomass and productivity in the Amazon River plume: correlation with seasonal river discharge. Continental Shelf Research 16, 291–319 (1996).
- Körtzinger, A. A significant CO2 sink in the tropical Atlantic Ocean associated with the Amazon River plume. Geophysical Research Letters 30, (2003).
- Goes, J. I. et al. Influence of the Amazon River discharge on the biogeography of phytoplankton communities in the western tropical north Atlantic. Progress in Oceanography 120, 29–40 (2014).
- Subramaniam, A. et al. Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean. Proceedings of the National Academy of Sciences 105, 10460–10465 (2008).
- Capone, D. G., Zehr, J. P., Paerl, H. W., Bergman, B. & Carpenter, E. J. Trichodesmium, a Globally Significant Marine Cyanobacterium. Science 276, 1221–1229 (1997).
- Carpenter, E. J. et al. Extensive bloom of a N2-fixing diatom/cyanobacterial association in the tropical Atlantic Ocean. Marine Ecology Progress Series 185, 273–283 (1999).
- Cooley, S. R. & Yager, P. L. Physical and biological contributions to the western tropical North Atlantic Ocean carbon sink formed by the Amazon River plume. Journal of Geophysical Research: Oceans 111, (2006).
- Coles, V. J. et al. The pathways and properties of the Amazon River Plume in the tropical North Atlantic Ocean: AMAZON RIVER PLUME. J. Geophys. Res. Oceans 118, 6894–6913 (2013).
- Gouveia, N. A., Gherardi, D. F. M. & Aragão, L. E. O. C. The Role of the Amazon River Plume on the Intensification of the Hydrological Cycle. Geophys. Res. Lett. 46, 12221–12229 (2019).
- del Giorgio, P. A., Cole, J. J. & Cimbleris, A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385, 148–151 (1997).
- le B. Williams, P. J. The balance of plankton respiration and photosynthesis in the open oceans. Nature 394, 55–57 (1998).
- Andreae, M. O. et al. Biogeochemical cycling of carbon, water, energy, trace gases, and aerosols in Amazonia: The LBA-EUSTACH experiments. Journal of Geophysical Research: Atmospheres 107, LBA 33-1-LBA 33-25 (2002).
- Liang, Y.-C. et al. Amplified seasonal cycle in hydroclimate over the Amazon river basin and its plume region. Nature Communications 11, 4390 (2020).
- Woodwell, G. M. et al. Global Deforestation: Contribution to Atmospheric Carbon Dioxide. Science 222, 1081–1086 (1983).
- Berenguer, E. et al. A large-scale field assessment of carbon stocks in human-modified tropical forests. Global Change Biology 20, 3713–3726 (2014).
- Booth, B. B. B. et al. High sensitivity of future global warming to land carbon cycle processes. Environ. Res. Lett. 7, 024002 (2012).
- Khanna, J., Medvigy, D., Fueglistaler, S. & Walko, R. Regional dry-season climate changes due to three decades of Amazonian deforestation. Nature Climate Change 7, 200–204 (2017).
- Gloor, M. et al. Intensification of the Amazon hydrological cycle over the last two decades. Geophysical Research Letters 40, 1729–1733 (2013).
- Timmermann, A. et al. Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 398, 694–697 (1999).
- Duffy, P. B., Brando, P., Asner, G. P. & Field, C. B. Projections of future meteorological drought and wet periods in the Amazon. PNAS 112, 13172–13177 (2015).
- Malhi, Y. et al. Climate Change, Deforestation, and the Fate of the Amazon. Science 319, 169–172 (2008).
- Jahfer, S., Vinayachandran, P. N. & Nanjundiah, R. S. Long-term impact of Amazon river runoff on northern hemispheric climate. Scientific Reports 7, 10989 (2017).