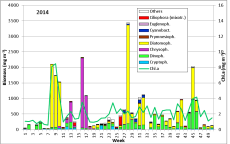
Phytoplankton development at the coastal station "Seebrücke Heiligendamm" in 2014
The Leibniz Institute for Baltic Sea Research conducts a coastal monitoring programme with weekly samplings at the jetty Heiligendamm (54°08,55' N; 11°50,60' E; 300 m off shore, 3 m water depth). The Department of Marine Biology analyses the surface samples, taken by means of a bucket, for phytoplankton composition and biomass and for chlorophyll a.
The phytoplankton biomass is determined by microscopical counting (UTERMÖHL method) and the chlorophyll a concentration by ethanol extraction and fluorometric measurement. Method instructions see http://helcom.fi/action-areas/monitoring-and-assessment/manuals-and-guidelines/combine-manual.
Phytoplankton counting was carried through by use of the counting programme OrgaCount and is based on the HELCOM-biovolume factors which are annually updated: http://www.ices.dk/marine-data/vocabularies/Documents/PEG_BVOL.zip (PEG_BVOL2013; basics see in Olenina et al. 2006). The analytical specifics of the chlorophyll a determination are published by Wasmund et al. (2006). According to the decision of the BLMP-subgroup “Quality Assurance” from 11.9.2008 we show here chlorophyll a data which are not corrected for pheopigments.

Microscopical analysis was not possible in three samples as they contained high sediment portions, caused by wind-induced sediment resuspension at the shallow station. The share of sediment-containing samples decreased in comparison with the previous year.
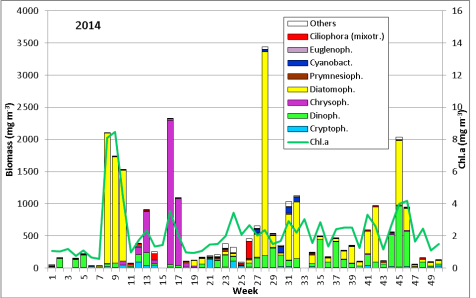
The results are shown in Fig. 1.
The phytoplankton biomass was low in January until mid of February 2014 while water temperature decreased from 5.1 to 1.8 °C. It was dominated by Ceratium tripos which originated from the previous autumn and survived the winter. Ceratium disappeared by the 11.2.14 (week 6) when salinity dropped to 11.6 PSU, indicating Baltic outflow. The phytoplankton biomass and chlorophyll-a concentrations fell below 42 mg/m3 and 0.7 mg/m3, respectively. Mainly cryptophyceae (Teleaulax spp., Plagioselmis prolonga, Hemiselmis sp.) remained.

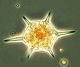
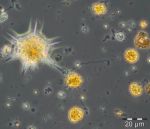
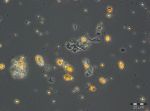
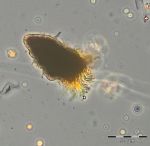
After an increase in water temperature and salinity to 2,9 °C and 15,3 PSU, respectively, by week 8 (25.2.2014) a sudden bloom of diatoms (Diatomophyceae) appeared, formed by Skeletonema marinoi (918 mg/m3), Thalassiosira spp. (403 mg/m3), Chaetoceros cf. wighamii (357 mg/m3, Image 1) and Detonula confervacea (211 mg/m3). This bloom was correlated with the chlorophyll-a concentration. It stayed until 12.3.2014 (week 10). Besides of nutrient exhaustion, also parasites may have played a role for the collapse of the bloom by week 11, as shown for Skeletonema (Image 2). However, Skeletonema marinoi survived longest in comparison with the other three taxa mentioned and accounted even for a biomass of 1219 mg/m3 on 12.3.2014 (week 10). The breakdown by the 18.3.2014 could be caused by strong winds (westerly wind of bf-4 still on the sampling day), but can also be an artefact due to many sediment particles in the sample, that mask cells and make counting results less reliable (Image 3).

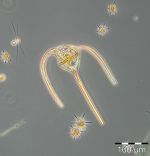
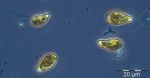
After the collapse of the diatom bloom, the flagellate Dictyocha speculum, counted to Chrysophyceae in Fig.1, developed strongly. In contrast to the two previous years, it formed a second spring bloom with a peak on 22.4.2014 (week 16; 2230 mg/m3). This species appears rarely with its typical silicon skeleton, but mainly in its „naked“ form (Image 4 and 5). Besides of this strong typical species of the late spring, other typical spring species could hardly grow in 2014. The dinoflagellates Ceratium tripos (Image 5) and Gyrodinium spirale disappeared after week 13, while the cryptophyceae Teleaulax spp. still remained in low biomass (Image 6).
The mixotrophic ciliate Mesodinium rubrum was still more degraded than in previous years, i.e. the decreasing tendency continues in Mecklenburg Bight. Also the euglenophyceae Eutreptiella braarudii, which was exceptionally strong in 2012, was unimportant in 2014, similar to 2013. The diatom Achnanthes taeniata, bloom-forming in 2011, was almost absent in 2014; it occurred only from 25.2. to 12.3.2014 in low biomass of about 20 mg/m3.
In summary, the spring bloom was clearly developed in 2014, with a typical first phase of diatoms and a second phase of flagellates. The diatom phase was dominated by Skeletonema marinoi as usual. The flagellate phase is made by different taxa in different years, mainly dinoflagellates, Dictyocha speculum or as exception Eutreptiella braarudii as well as the ciliate Mesodinium rubrum. In 2014, Dictyocha speculum dominated clearly.
From week 18 to 19 (6.5. to 13.5.2014), Dictyocha speculum disappeared almost completely, and the diatoms Rhizosolenia setigera (on 13.5.2014) and Skeletonema marinoi (on 20.5.2014) became dominant for a short while. On 27.5.2014 (week 21), cryptophyceae (Plagioselmis prolonga, Teleaulax spp., Hemiselmis sp.) were abundant.
Ceratium tripos, already shown in Image 5, gained dominance on 3.6. and 10.6.2014 (week 22 and 23) because this dinoflagellate, forming blooms in autumn, starts its development already in late spring. Besides this, many small flagellates as well as small (2-5 µm) unidentified single cells occurred (Image 7). They are hard to find and probably underestimated at the biomass minimum on 24.6.2014. The chlorophyll data don’t show such a strong minimum, because these small cells may be especially rich in chlorophyll.
After a short appearance of Mesodinium rubrum in week 26, the typical large-celled diatoms, the already mentioned Ceratium tripos and nitrogen-fixing cyanobacteria developed continuously. The dominating diatom species of the summer bloom change from year to year. The bloom-forming species in 2014, just like in 2013, was Dactyliosolen fragilissimus. It reached its maximum with 3111 mg/m3 already on 15.7.2014 (Week 28). Ceratium tripos showed an intermittent maximum of 195 mg/m3 one week later. Also on 22.7.2014, the rarer mixotrophe ciliate Laboea strobila (Image 8) was present.

A further week later (week 30), cyanobacteria appeared with 86 mg/m3. They were mainly represented by Nodularia spumigena (Image 9) and to a smaller extent by Aphanizomenon sp. (Image 10). Cyanobacteria biomass is usually low in Mecklenburg Bight. The extreme appearance of Nodularia spumigena (2060 mg/m3) in 2013 has to be considered as exception.

Dactyliosolen fragilissimus was again strongly present from 5.8. to 12.8.2014 (weeks 31 and 32). On 19.8.2014, westerly winds of bf-4 resuspended much sediment, making the sample non-analysable. After this, Dactyliosolen fragilissimus was absent. The most important diatoms in week 34 were Coscinodiscus granii and Pseudo-nitzschia pungens.
The following period was characterized by Ceratium tripos (e.g. 352 mg/m3 in week 35). Later, diatoms became dominant, as for example in week 38 Coscinodiscus granii (66 mg/m3) and Dactyliosolen fragilissimus (25 mg/m3) and in week 39-41 Coscinodiscus radiatus (up to 33 mg/m3) and Dactyliosolen fragilissimus (up to 124 mg/m3). Cerataulina pelagica developed strongly by the 21.10.2014 (week 42; 810 mg/m3). It was still dominant on 11.11.2014 (week 45) with 847 mg/m3, besides Ceratium tripos (558 mg/m3). The relatively high biomass of the heterotrophic dinoflagellate Polykrikos schwartzii (265 mg/m3) on this day is astonishing. During the late phase of this autumn bloom there was nearly a biomass balance between Ceratium species (C. tripos, C. fusus, C. lineatum) and Cerataulina pelagica. The biomass was very low in the following 47. week (25.11.2014) and it was not recovering significantly during the rest of the year.
We may conclude in a long-term comparison, that a clear autumn bloom occurred in the year 2014, in contrast to 2013. The autumn was mainly formed by the typical dinoflagellate Ceratium and by the diatom Cerataulina pelagica. The diatom Coscinodiscus granii, originating from the central Baltic Sea, occurred in 2014 only rarely, which is a sign for only little outflow from the Baltic Sea in autumn 2014.
The autumn blooms have reduced since 2011 and appeared earlier in comparison with the years 2008 und 2009. In 2014, the autumn bloom had a „normal“ occurrence in November. In the years 2008 and 2009 strong diatom blooms occurred as late as Dezember which led us to the hypothesis, that the vegetation period extends. This tendency has not continued. In 2014, the vegetation period lasted only from 25.2. to 18.11.2014.
Acknowledgement
We thank the colleagues of the department Marine Chemistry for their cooperation and especially the colleagues of the department Biological Oceanography, Regina Hansen, Anja Hansen and Annett Grüttmüller, for their participation in the sampling activities. We acknowledge also the technical conditions for the data work and the loading of this report to the IOW site by Solvey Hölzel, Dr. Steffen Bock und Dr. Susanne Feistel.
References:
Olenina, I., Hajdu, S., Andersson, A.,Edler, L., Wasmund, N., Busch, S., Göbel, J., Gromisz, S., Huseby, S., Huttunen, M., Jaanus, A., Kokkonen, P., Ledaine, I., Niemkiewicz, E. (2006): Biovolumes and size-classes of phytoplankton in the Baltic Sea. Baltic Sea Environment Proceedings No.106, 144pp.
http://www.helcom.fi/Lists/Publications/BSEP106.pdf
Wasmund, N., Topp, I., Schories, D. (2006): Optimising the storage and extraction of chlorophyll samples. Oceanologia 48: 125-144.
IOW, 02.02.2015
Dr. Norbert Wasmund,
Christian Burmeister,
Susanne Busch.
Leibniz Institute for Baltic Sea Research Warnemünde (IOW),
Seestr. 15,
D-18119 Rostock-Warnemünde
Corresponding author: Dr. Norbert Wasmund

State of the Baltic Sea
- Annual Reports on the state of the Baltic Sea Environment
- Cruise Reports
- Data from the autonomous measuring stations
- Development of the suboxic and anoxic regions since 1969
- Baltic Thalweg transect since 2014
- Algal blooms at Heiligendamm since 1998
- "Major Baltic Inflow" December 2014
- "Major Baltic Inflow" January 2003
- Baltic saline barotropic inflows 1887 - 2018
- Further Reading