Phytoplankton development at the coastal station “Seebrücke Heiligendamm” in 2016
The departments of Marine Chemistry and of Biological Oceanography of the Leibniz Institute for Baltic Sea Research conduct a coastal monitoring programme with weekly samplings at the jetty Heiligendamm (54°08,76' N; 11°50,58' E; nearly 3 m water depth). This report presents the results of the investigations on phytoplankton composition and biomass and on chlorophyll a.
Surface samples were taken by means of a bucket. The phytoplankton biomass is determined by microscopical counting (UTERMÖHL method) and the chlorophyll a concentration by ethanol extraction and fluorometric measurement. Method instructions see http://www.helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Guidelines%20for%20monitoring%20phytoplankton%20species%20composition,%20abundance%20and%20biomass.pdf.
Phytoplankton counting was carried through by use of the counting programme OrgaCount and is based on the HELCOM-biovolume factors which are annually updated: http://www.ices.dk/marine-data/vocabularies/Documents/PEG_BVOL.zip (basics see in Olenina et al. 2006). The analytical specifics of the chlorophyll a determination are published by Wasmund et al. (2006). According to the decision of the BLMP-subgroup “Quality Assurance” from 11.9.2008 we show here chlorophyll a data which are not corrected for pheopigments.

Microscopical analysis was not possible in 9 samples as they contained high sediment portions, caused by wind-induced sediment resuspension at the shallow station. These samples originated mainly from periods of low biomass; therefore the loss of information was limited.
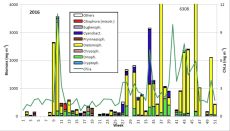
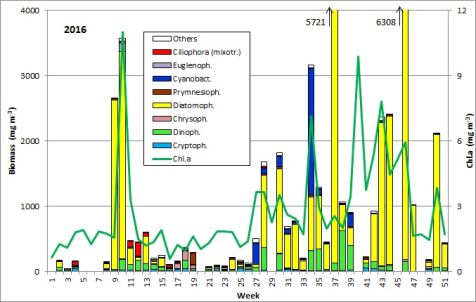
The results are shown in Fig. 1.

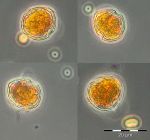
The phytoplankton biomass was low until week 8 (23.2.2016). The development of diatoms (Diatomophyceae: Skeletonema marinoi) started on 23.2.2016. The high diatom biomass in the following week was, however, not based on growth of this species, but on inflow of a water body, characterized be high salinity (17.3 psu) and the large diatom Coscinodiscus concinnus (2361 mg/m3), that is poor in chlorophyll. This water body disappeared by week 10 (8.3.2016) and was replaced by a water body of lower salinity (12.9 psu), containing a bloom of Skeletonema marinoi (3022 mg/m3 (Image 1). This small-celled diatom is rich in chlorophyll, causing the annual maximum of chlorophyll-a concentration of 11.0 mg/m3. Besides of Skeletonema marinoi, diatoms of the genus Chaetoceros (e.g. Ch. wighamii), dinoflagellates (e.g. Katodinium rotundatum) and the Euglenophycee Eutreptiella braarudii (Image 1) occurred in significant biomasses. Thus, this spring bloom appeared two weeks later than in the previous year and it was much shorter. The rapid disappearance by week 11 (15.3.2016) may be explained by replacement of the water body, as salinity decreased to 9.2 psu. In week 11, the biomass of Skeletonema marinoi amounted only to 200 mg/m3, but the mixotrophic ciliate Mesodinium rubrum appeared with 87 mg/m3.
The biomass of Mesodinium rubrum increased by week 12 to 198 mg/m3, but stayed much lower than usual for this species. Also the Dictyochophyceen (Dictyocha speculum, Verrucophora farcimen; in Fig. 1 shown as „Chrysophyceae“) were insignificant in comparison with some earlier years. Coscinodiscus concinnus and Pseudo-nitzschia seriata occurred on 29.3.2016 (week 13; Image 2). The latter is unusual for spring, but it stayed until 12.4.2016; this genus appeared again in autumn.
The biomass was very low in weeks 14 and 15, but the chlorophyll-a concentrations were relatively high, probably because the dominating flagellates were rich in chlorophyll, like Katodinium rotundatum, Plagioselmis prolonga, Dinobryon balticum, Teleaulax, Eutreptiella and Hemiselmis (the latter only in week 14). In week 15, Pseudo-nitzschia was dominating.
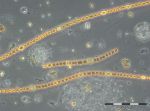
After the minimum in biomass on 19.4.2017 (week 16), the following flagellates developed: Dictyocha speculum (maximum in week 18: 135 mg/m3; see Image 3), Peridiniella danica (only in week 18: 119 mg/m3; see Image 4) and unidentified Prymnesiales (maximum in week 19: 173 mg/m3).

Consequently, the spring bloom from 2016 was a typical diatom bloom. It occurred rather late and was of unusually short duration. The change of species during the bloom might misleadingly be interpreted as a true succession, but in reality a marine water body, characterized by Coscinodiscus concinnus was replaced by a brackish water body dominated by Skeletonema marinoi. Typical species succeeding the diatoms, like Mesodinium rubrum, Dictyocha speculum, Eutreptiella braarudii as well as Dinophyceae and Prymnesiophyceae were poorly developed in 2016.
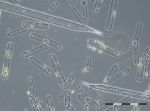
The summer season started after a short data gap with low biomass (78 mg/m3, chlorophyll-a concentration 1,3 mg/m3) in week 21 (24.5.2016). The carbon/chl.a ratio was very low (16.5), which is close to the lower border of the natural range, that reaches from 10 to 120 according to literature (see Wasmund et al. 2016). Cryptophyceae, Gymnodiniales and Prymnesiales dominated from week 21 to 23. In week 24 (14.6.2016), the diatom Actinocyclus sp. occurred with 82 mg/m3, but it disappeared in the course oft he following three weeks while Dactyliosolen fragilissimus developed continuously.
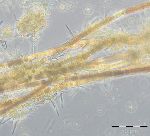
On 5.7.2016 (week 27), the nitrogen-fixing cyanobacterium Dolichospermum sp. appeared suddenly with 261 mg/m3 (Image 5), accompanied by Aphanizomenon sp. (19 mg/m3) and Nodularia spumigena (only 7 mg/m3). The cyanobacteria decreased in weeks 28 to 30, but a diatom bloom developed which started with Dactyliosolen fragilissimus (704 mg/m3; 12.7.2016 ) and Skeletonema marinoi (185 mg/m3) and continued with Proboscia alata (1086 mg/m3; 26.7.2016; Image 6).
The bloom was dominated by of Dactyliosolen fragilissimus from 2.8.2016 (week 31) to 27.9.2016 (week 39) with an extreme maximum of this species of 3448 mg/m3 on 13.9.2016 (Image 7). Also Pseudosolenia calcar-avis was strongly developed with a maximum of 1300 mg/m3 on 13.9.2016.
The short but extremely high cyanobacterium peak on 23.8.2016 is of special interest as it is very unusual in this region. It is formed by Nodularia spumigena, which seems to be aged already as it is covered by Nitzschia paleacea (Image 8). Such massive cyanobacteria blooms provoke strong public interest (e.g. article by Maria Pistor in the newspaper „Norddeutsche Neueste Nachrichten“ from 23.8.2016). A similar strong but short bloom was noticed in 2013. At the same time, diverse dinoflagellates occur (Ceratium tripos, Prorocentrum cordatum, Prorocentrum micans, Alexandrium pseudogonyaulax and unidentified Gymnodiniales). The typical bloom-forming diatom of autumn in the Baltic Proper, Coscinodiscus granii, was present only from 30.8.2016 (week 35) to 20.9.2016 (week 38) and in relatively low biomass up to 86 mg/m3. Cyanobacteria (Nodularia spumigena, Aphanizomenon) appeared again in week 39 (27.9.2016).

A huge bloom of the diatom Cerataulina pelagica was present from 11.10.2016 (week 41) to 15.11.2016 (week 46) with a peak biomass of 5487 mg/m3 (Image 9). It was accompanied by the diatoms Skeletonema marinoi, Pseudosolenia calcar-avis, Ditylum brightwellii, Rhizosolenia pungens, Guinardia delicatula, Chaetoceros spp., Pseudo-nitzschia spp., Thalassiosira spp. and a decreasing population of Dactyliosolen fragilissimus (Image 9). Moreover, the flagellates Dictyocha speculum, Hemiselmis sp., Pyramimonas sp., Teleaulax spp., Ceratium fusus as well as the ciliate Mesodinium rubrum occurred in low biomasses.
The usually bloom-forming dinoflagellate Ceratium tripos was lacking in autumn 2016. Only Ceratium fusus occurred on 20.9.2016 with a biomass of 192 mg/m3 whereas Ceratium tripos was present with a similar biomass as early as 23.8.2016. High biomass, dominated by Cerataulina pelagica (945 mg/m3), Pseudo-nitzschia spp. (240 mg/m3) and Rhizosolenia setigera (230 mg/m3), were found by the 13.12.2016 (week 50).
In summary, the spring bloom 2016 was a almost pure diatom bloom. Other phytoplankton groups were unimportant in spring. In contrast to 2015, a strong diatom bloom and a short cyanobacteria bloom was present in summer 2016. Significant cyanobacteria biomass occurred still by the end of September. The typical dinoflagellate genus of the autumn, Ceratium, was already poor in 2015. The autumn bloom was long-lasting and was clearly dominated by diatoms. Perhaps a trend to long diatom autumn blooms will establish.
Fig. 1: Composition of the phytoplankton biomass and concentration of chlorophyll a from 6.1. to 20.12.2016 at the jetty Heiligendamm
Acknowledgement
We wish to thank the colleagues of the department Marine Chemistry for their cooperation and especially the colleagues of the department Biological Oceanography, Regina Hansen and Annett Grüttmüller, for their participation in the sampling activities. We acknowledge also the technical conditions for the data work and the loading of this report to the IOW site by Solvey Hölzel, Dr. Steffen Bock und Dr. Susanne Feistel.
References:
Olenina, I., Hajdu, S., Andersson, A.,Edler, L., Wasmund, N., Busch, S., Göbel, J., Gromisz, S., Huseby, S., Huttunen, M., Jaanus, A., Kokkonen, P., Ledaine, I., Niemkiewicz, E. (2006): Biovolumes and size-classes of phytoplankton in the Baltic Sea. Baltic Sea Environment Proceedings No.106, 144pp.
http://www.helcom.fi/Lists/Publications/BSEP106.pdf
Wasmund, N., Topp, I., Schories, D. (2006): Optimising the storage and extraction of chlorophyll samples. Oceanologia 48: 125-144.
Wasmund, N., Siegel, H., Bohata, K., Flohr, A., Hansen, A., Mohrholz, V. (2015): Phytoplankton stimulation in frontal regions of Benguela upwelling filaments by internal factors. Frontiers in Marine Science 3 (210): 1-17. doi: 10.3389/fmars.2016.00210.
IOW, 08.06.2017
Dr. Norbert Wasmund,
Susanne Busch,
Christian Burmeister.
Leibniz Institute for Baltic Sea Research Warnemünde (IOW),
Seestr. 15,
D-18119 Rostock-Warnemünde
Corresponding author: Dr. Norbert Wasmund

State of the Baltic Sea
- Annual Reports on the state of the Baltic Sea Environment
- Cruise Reports
- Data from the autonomous measuring stations
- Development of the suboxic and anoxic regions since 1969
- Baltic Thalweg transect since 2014
- Algal blooms at Heiligendamm since 1998
- "Major Baltic Inflow" December 2014
- "Major Baltic Inflow" January 2003
- Baltic saline barotropic inflows 1887 - 2018
- Further Reading