Phytoplankton development at the coastal station “Seebrücke Heiligendamm” in 2017
The departments of Marine Chemistry and of Biological Oceanography of the Leibniz Institute for Baltic Sea Research conduct a coastal monitoring programme with weekly samplings at the jetty Heiligendamm (54°08,76' N; 11°50,58' E; nearly 3 m water depth). This report presents the results of the investigations on phytoplankton composition and biomass and on chlorophyll a.
Surface samples were taken by means of a bucket. The phytoplankton biomass is determined by microscopical counting (UTERMÖHL method) and the chlorophyll a concentration by ethanol extraction and fluorometric measurement. Method instructions see http://www.helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Guidelines%20for%20monitoring%20phytoplankton%20species%20composition,%20abundance%20and%20biomass.pdf.
Phytoplankton counting was carried through by use of the counting programme OrgaCount and is based on the HELCOM-biovolume factors which are annually updated: http://www.ices.dk/marine-data/vocabularies/Documents/PEG_BVOL.zip (basics see in Olenina et al. 2006). The analytical specifics of the chlorophyll a determination are published by Wasmund et al. (2006). According to the decision of the BLMP-subgroup “Quality Assurance” from 11.9.2008 we show here chlorophyll a data which are not corrected for pheopigments.

Microscopical analysis was not possible in 13 samples as they contained high sediment portions, caused by wind-induced sediment resuspension at the shallow station. The chlorophyll analyses were still possible and allowed information on phytoplankton biomass even if phytoplankton data were lacking.
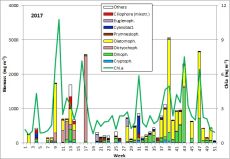
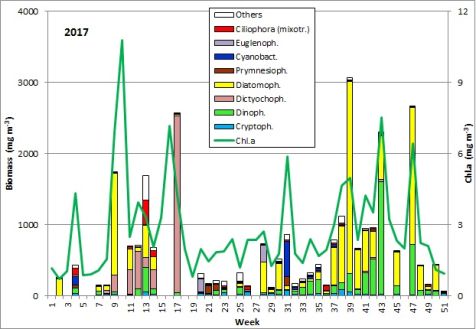
The results are shown in Fig. 1.
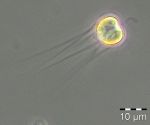
At the beginning of the year 2017, the diatom Rhizosolenia setigera was still dominant. It was present on 13.12.2016 with a biomass of 230 mg/m3 and kept a unusually high biomass of 133 mg/m3 by 10.1.2017 (week 2). More unusual for this season were the high chlorophyll concentration and the diverse phytoplankton on 24.1.2017 (week 4). Especially the high biomass (125 mg/m3) of the cyanobacterium Aphanizomenon sp. (Image 1) was astonishing. This genus forms blooms only in summer preferably in the northern parts of the Baltic Proper. Now it appeared widely without heterocytes, but it had the typical taxon-specific hyaline end-cells. The problems with species identification are known (cf. Laamanen 2002), that’s why the species is called „Aphanizomenon sp.“. The lack of heterocytes at presence of nitrate (3.6 µM on 24.1.2017) was frequently reported (Laamanen 1996, Adams & Duggan 1999, Palińska & Surosz 2008). The drop in salinity from 17.5 psu on 10.1.2017 to 10.3 psu on 24.1.2017 could be an evidence of freshwater inflow from Lake Conventer See, which may have contained the high Aphanizomenon-biomass. In that case, this would not be the brackish-water species of the Baltic Sea, but the true fresh-water species Aphanizomenon flos-aquae. However, the lack of accompanying fresh-water species would negate the hypothesis on fresh-water inflow although they could have died when entering the brackish water whereas Aphanizomenon sp. tolerates brackish conditions. Important accompanying species were Mesodinium rubrum (41 mg/m3), Heterocapsa rotundata (41 mg/m3), Prymnesiales (38 mg/m3), Teleaulax spp. (21 mg/m3) and Eutreptiella spp. (21 mg/m3). Aphanizomenon sp. was already absent in the quantitatively non-evaluable sample from 31.1.2017 and the chlorophyll content was low which indicates that the assumed fresh-water inflow was only of short duration.
Starting from a relatively low biomass on 21.2.2017 (week 8), a massive growth occurred within one week. Species that had dominated already on 21.2.2017, Skeletonema marinoi (52 mg/m3) and Dictyocha speculum (42 mg/m3), grew to a biomass of 1303 mg/m3 and 238 mg/m3, respectively, by 28.2.2017 (week 9), which indicates almost exponential growth. As mentioned in our previous reports, we have to admit that such strong changes in biomass may not only be caused by growth within the water body, but also by advection of another water body to our sampling station.
The maximum of the spring bloom is expected on 7.3.2017 (week 10) according to the chlorophyll-peak of 10.8 mg/m3. Unfortunately, the phytoplankton sample from 7.3.2017 was microscopically not evaluable. During the final phase of this diatom bloom, Skeletonema marinoi declined whereas Dictyocha speculum increased.
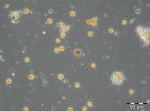
A clearly deviating species composition appeared on 28.3.2017 (week 13). Besides of Skeletonema marinoi (407 mg/m3) and Dictyocha speculum (136 mg/m3), high biomasses of the ciliate Mesodinium rubrum (343 mg/m3), the dinoflagellate Peridiniella danica (233 mg/m3) and the chrysophyte Apedinella radians (313 mg/m³) occurred. Before this report, Dictyocha speculum was counted to the Chrysophyceae in the Figure 1, although this species was, besides others, separated by taxonomists as an own class „Dictyochophyceae“ many years ago. Dictyocha speculum was much more abundant than the remaining Chrysophyceae in the past. Therefore we decided starting this year with a separate category „Dictyochophyceae“ and putting the remaining Chrysophyceae into the „Others“. Strangely, just in 2017 (but only on 28.3.2017), the Chrysophyceae with Apedinella radians became abundant (Image 3).

Successively, Dictyocha speculum (Image 4) became dominant and reached an extreme maximum of 2487 mg/m3 on 25.4.2017 (week 17). The chlorophyll data showed the maximum of this post-spring bloom already on 18.4.2017 (week 16), unfortunately again without evaluable phytoplankton sample. On that day, phosphate concentrations dropped to a minimum of 0.03 µM and recovered in the following weeks. The concentrations in nitrate and nitrite dropped to 0.05 µM by 16.5.2017 (week 20). On that day, Eutreptiella sp. dominated shortly with 179 mg/m3. Moreover, the cryptophytes Teleaulax sp. and Plagioselmis prolonga as well as the dinoflagellate Heterocapsa rotundata were worth mentioning. By this stage, the spring bloom had finished. A post-spring bloom of Dictyocha speculum after the „classical“ diatom spring bloom was found in several years and is typical for this sea area.
During the summer minimum from week 21 to 26, nitrate+nitrite concentrations were < 0,08 µM (except one outlier) and phytoplankton biomass was < 250 mg/m3. Unidentified mixotrophic Prymnesiales, mostly in size class 2-4 µm, dominated at the beginning (115 mg/m3). They were more and more replaced by Gymnodiniales and later by Plagioselmis prolonga, Mesodinium rubrum and Heterocapsa rotundata. On 20.6.2017 (week 25), the heterotrophic nanoflagellates Telonema sp. and Katablepharis remigera (in Fig. 1 as „Others“) became dominant.
The nitrate concentrations have regenerated (0.79 µM) by 4.7.2017 (week 27), perhaps only caused by wind-induced resuspension (wind force 6), since the plankton sample was not evaluable due to a high sediment portion. After the strong north-western wind, combined with salt water inflow (salinity on 4.7.2017: 12.7 psu), the typical summer diatom Dactyliosolen fragilissimus (410 mg/m3) and the euglenophyte Eutreptiella sp. (230 mg/m3) appeared on 11.7.2017 (week 28). The following low biomass value (week 29) is probably caused by reduced quantification due to overlaying sediment particles. At least, Dactyliosolen fragilissimus was still dominant on 25.7.2018 (week 30) with 351 mg/m3.
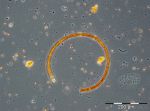
Dactyliosolen fragilissimus has almost completely disappeared by 1.8.2017 (week 31) whereas the nitrogen-fixing cyanobacteria Aphanizomenon sp. (221 mg/m3; Image 5) and Nodularia spumigena (228 mg/m3; Image 6) appeared. This cyanobacteria bloom was unusually strong for this sea area, but only of short duration, as already found in 2016. On 8.8.2017 (week 32), cyanobacteria were negligible. In the following three weeks (weeks 33-35), Ceratium tripos, Pseudosolenia calcar-avis, Alexandrium pseudogonyaulax, Prorocentrum cordatum and Prorocentrum micans grew slowly. Starting from 12.9.2017 (week 37) a diatom bloom developed again. It was characterized by Dactyliosolen fragilissimus on 12.9.2017 (374 mg/m3), but on 19.9.2017 mainly by Cerataulina pelagica (419 mg/m3) and Coscinodiscus granii (308 mg/m3). The peak of this diatom bloom was reached on 27.9.2017 (week 39) with clear dominance of Coscinodiscus granii (2451 mg/m3) and a sub-dominance of Ceratium tripos (213 mg/m3); Image 7.

Coscinodiscus granii and Ceratium tripos are typical species of the autumn bloom, but they were almost not present in 2016. In the last report, we hypothesized on a long-term decrease of Ceratium. However, in 2017, Ceratium tripos regained its usually strong presence. It reached its peak on 24.10.2017 (week 43) with a biomass of 1129 mg/m3. In contrast, Coscinodiscus granii has completely disappeared by 24.10.2017.
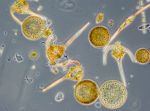
As a replace of Coscinodiscus granii, the potentially toxic diatom genus Pseudo-nitzschia grew strongly. It reached its peak on 21.11.2017 (week 47) with a biomass of 1221 mg/m3 (Image 8). Electron-microscopic analyses revealed that Pseudo-nitzschia pseudodelicatissima was the main species whereas Pseudo-nitzschia pungens was sub-dominant (Image 9). A bloom of this genus is rare in the western Baltic. Pseudo-nitzschia was present by the 12.12.2017 (week 50) while other diatom species became already stronger. Rhizosolenia setigera and Thalassiosira spp. reached biomasses > 70 mg/m3 on that day.

In summary, we may emphasise the unusual appearance of the cyanobacterium Aphanizomenon sp. already in January. The spring bloom 2017 consisted of two successional phases, which is rather common. The first phase was a clear diatom bloom (Skeletonema marinoi), while the second is usually made by flagellates. This flagellate phase was characterized, as in many previous years, by the naked forms of Dictyocha speculum. In summer 2017, a moderate diatom bloom (Dactyliosolen fragilissimus) and a relatively strong but short cyanobacteria bloom was realised. Low cyanobacteria biomasses were present even by the mid of September. The long-lasting autumn bloom was dominated by diatoms (Coscinodiscus granii, followed by Pseudo-nitzschia pseudodelicatissima). Also the typical dinoflagellate genus Ceratium was in contrast to previous years again bloom-forming.
Fig. 1: Composition of the phytoplankton biomass and concentration of chlorophyll a from 3.1. to 19.12.2017 at the jetty Heiligendamm.
Acknowledgement
We wish to thank the colleagues of the department Marine Chemistry (Lars Kreuzer, Jenny Jeschek, Birgit Sadkowiak), who accompanied the joint sampling activities and analysed the nutrients, as well as the colleagues of the IT Group (Dr. Steffen Bock, Dr. Susanne Feistel, Solvey Hölzel), who processed the data for storage in the data base and loaded this report to the IOW homepage.
References:
Adams, D.G., Duggan, P.S. (1999): Tansley Review No. 107: Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 144: 3-33.
Laamanen M. (1996): Cyanoprokaryotes in the Baltic Sea ice and winter plankton. Algological Studies 83: 423-433.
Laamanen M. (2002): Genetic and species diversity of planktonic cyanobacteria in the northern Baltic Sea. Finnish Institute of Marine Research, Contributions No. 4.
Olenina, I., Hajdu, S., Andersson, A.,Edler, L., Wasmund, N., Busch, S., Göbel, J., Gromisz, S., Huseby, S., Huttunen, M., Jaanus, A., Kokkonen, P., Ledaine, I., Niemkiewicz, E. (2006): Biovolumes and size-classes of phytoplankton in the Baltic Sea. Baltic Sea Environment Proceedings No.106, 144pp.
http://www.helcom.fi/Lists/Publications/BSEP106.pdf
Palińska, K.A., Surosz, W. (2008): Population of Aphanizomenon from the Gulf of Gdańsk (Southern Baltic Sea): differences in phenotypic and genotypic characteristics. Hydrobiologia 607: 163-173.
Wasmund, N., Topp, I., Schories, D. (2006): Optimising the storage and extraction of chlorophyll samples. Oceanologia 48: 125-144.
Wasmund, N., Siegel, H., Bohata, K., Flohr, A., Hansen, A., Mohrholz, V. (2015): Phytoplankton stimulation in frontal regions of Benguela upwelling filaments by internal factors. Frontiers in Marine Science 3 (210): 1-17. doi: 10.3389/fmars.2016.00210.
IOW, 08.06.2018
Dr. Norbert Wasmund,
Susanne Busch,
Christian Burmeister
Regina Hansen.
Leibniz Institute for Baltic Sea Research Warnemünde (IOW),
Seestr. 15,
D-18119 Rostock-Warnemünde
Corresponding author: Dr. Norbert Wasmund

State of the Baltic Sea
- Annual Reports on the state of the Baltic Sea Environment
- Cruise Reports
- Data from the autonomous measuring stations
- Development of the suboxic and anoxic regions since 1969
- Baltic Thalweg transect since 2014
- Algal blooms at Heiligendamm since 1998
- "Major Baltic Inflow" December 2014
- "Major Baltic Inflow" January 2003
- Baltic saline barotropic inflows 1887 - 2018
- Further Reading